Toe Implants

- Screw based implants provide rock solid fixation
- HemiCAP DF® dual implant curvatures improve dorsal role-off and prevent osteophyte regrowth
- ToeMotion™ screw-in baseplate and modular poly inserts provides optimal intraoperative flexibility
- Super smooth articulating cobalt chrome surface to minimize wear on the opposing side
- Conical shaped screw to optimize bone-screw interface
- Inlay arthroplasty and minimal bone removal allows for future fusion if necessary – “No Bridges Burned”
- Anatomic inlay of Toe Classic HemiCAP® maintains the length of the lesser metatarsals
- Standardized thread pitch for precise depth and decompression
- Proven clinical history with over 20,000 MTP implants
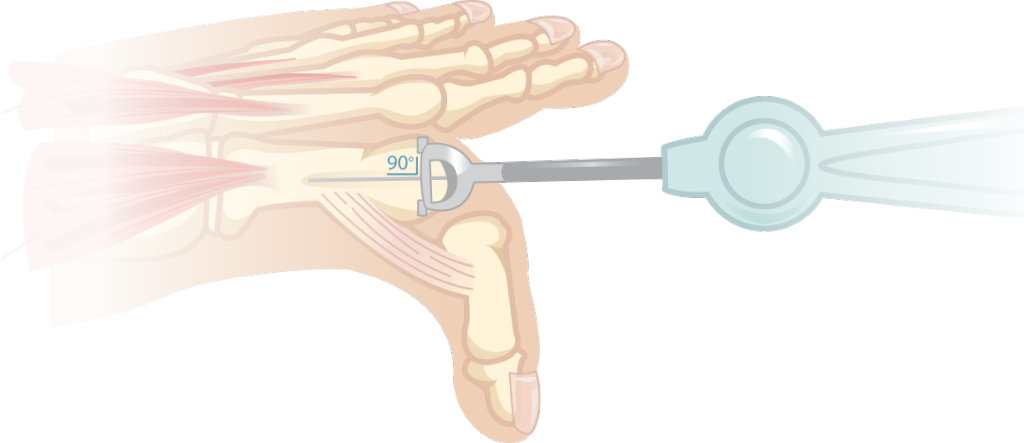 The Toe HemiCAP® implant system is comprised of two parts, an articular cap and a fixation component. The instruments are organized in the order of surgery, proceeding from left to right and top to bottom. The procedure begins with a guide to identify the size of the metatarsal head. Then place the guide wire perpendicular to the head. A step drill is used to prepare the screw hole before the fixation component is advanced (joint decompression can be adjusted by advancing the screw 2-4mm). The uniquely designed instruments are used to map the contours of the patient’s native surface curvatures using the fixation component as a central axis. A reamer then removes the damaged cartilage to create a socket for the implant. If using the DF HemiCAP®, a second reamer is inserted into the screw to ream the dorsal socket. Place the trial to assess proper implant fit. After, any osteophytes or boney edges are trimmed off around the implant so bone and cartilage surfaces surrounding the implant are flush. (If you are using the ToeMotino Total Toe System, skip impacting the DF implant and contiune on to the next paragraph). If you are only implanting the DF HemiCAP®, once the surface preparation is complete, the implant is positioned and seated with several taps of the mallet. The ToeMotion™ phalangeal component is prepared in a similar manner. A guidewire is placed perpendicular to the phalangeal surface. A reamer is then used to prepare a socket until the reamer is slightly recessed to the surrounding surface. The sutures of the sterile delivery tool are then placed behind the phalangeal component and this metal baseplate is then screwed into place so that the final implant is slightly recessed. Plastic trials are then placed into the baseplate and a range of motion is performed to assess which insert is best for the patient. Once the proper insert is chosen it is placed into baseplate, the delivery tool is then cinched down against the implant and the lead sutures are then twisted until an audible click is heard which means the poly has locked into the baseplate. The DF HemiCAP® is then impacted onto the metatarsal taper post to lock the morse taper. The wound is then closed.
The Toe HemiCAP® implant system is comprised of two parts, an articular cap and a fixation component. The instruments are organized in the order of surgery, proceeding from left to right and top to bottom. The procedure begins with a guide to identify the size of the metatarsal head. Then place the guide wire perpendicular to the head. A step drill is used to prepare the screw hole before the fixation component is advanced (joint decompression can be adjusted by advancing the screw 2-4mm). The uniquely designed instruments are used to map the contours of the patient’s native surface curvatures using the fixation component as a central axis. A reamer then removes the damaged cartilage to create a socket for the implant. If using the DF HemiCAP®, a second reamer is inserted into the screw to ream the dorsal socket. Place the trial to assess proper implant fit. After, any osteophytes or boney edges are trimmed off around the implant so bone and cartilage surfaces surrounding the implant are flush. (If you are using the ToeMotino Total Toe System, skip impacting the DF implant and contiune on to the next paragraph). If you are only implanting the DF HemiCAP®, once the surface preparation is complete, the implant is positioned and seated with several taps of the mallet. The ToeMotion™ phalangeal component is prepared in a similar manner. A guidewire is placed perpendicular to the phalangeal surface. A reamer is then used to prepare a socket until the reamer is slightly recessed to the surrounding surface. The sutures of the sterile delivery tool are then placed behind the phalangeal component and this metal baseplate is then screwed into place so that the final implant is slightly recessed. Plastic trials are then placed into the baseplate and a range of motion is performed to assess which insert is best for the patient. Once the proper insert is chosen it is placed into baseplate, the delivery tool is then cinched down against the implant and the lead sutures are then twisted until an audible click is heard which means the poly has locked into the baseplate. The DF HemiCAP® is then impacted onto the metatarsal taper post to lock the morse taper. The wound is then closed.
 HemiCAP® technology was designed so patients can continue working and retain an active lifestyle without compromising future treatments.
HemiCAP® technology was designed so patients can continue working and retain an active lifestyle without compromising future treatments.
- May be performed on an outpatient basis
- Allows for preservation of bone and soft tissues
- Bridges the gap between biological therapies and joint fusion
- Clinical studies demonstrate positive clinical outcomes at 5-7 years
- Maintains existing joint biomechanics thereby allowing normal motion
- Patients experience a rapid return to activity for both work and exercise
- Patients report pain relief, rapid recovery and range of motion improvements

- The HemiCAP® procedure is intuitive and easy to learn
- The minimally invasive outpatient procedure typically takes less than an hour
- Soft-tissue envelope and native joint biomechanics are maintained
- Skeletal anatomy and bone stock are preserved allowing future fusion or total toe
- Phalangeal surface reamer allows for a controlled chielectomy
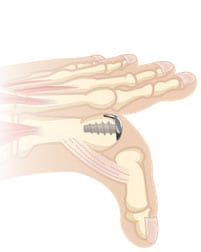
- The two components are connected together via morse taper, with zero reported loosening
Toe HemiCAP® systems are approved for use in the USA, CE Marked countries and many other markets around the world.
The ToeMotion Total Toe is approved for use in the USA only at this time.
[/fourcol_three][fourcol_one_last]


Download the
Toe Brochure
[/fourcol_one_last]
NEWs / updates / blog
[subscribe-box]
[whats-new-patients]